
The image above is a still from Just Imagine, a 1930 movie about what life in New York City would be like in the year 1980.
This series of pieces on the disaster of COVID-19 (the virus and the modRNA “vaccines” for said virus) is dedicated to the memory of Yours Truly’s cousin Bill, who “died suddenly and unexpectedly” in September, 2023. May he rest in eternal Peace.
Three prefatory notes: One, that what is presented here is only “scratching the surface” regarding the substitution of “Process 2” for the original “Process 1” manufacturing method for the Pfizer-BioNTech “flagship” COVID-19 “vaccine”, BNT162b2; Two, the exact and complete details of “Process 2” are likely a “trade secret” to the company (except that a full description may be in a document given by said company to the FDA, and which was “redacted out” in case it ever got published under FOIA); and, Three, that every Pfizer-BioNTech COVID-19 “vaccine” since October, 2020, has been made using “Process 2” — including the “latest” version, the “2023-2024 Formula” version for use against the (now basically obsolete) XBB.1.5 Omicron variant.
The trail in regards a discussion of the sudden change from the original “Process 1” to the substituted “Process 2” for BNT162b2 can potentially begin in several places; for purposes of today’s presentation, it will begin here: www.bmj.com/content/378/bmj.o1731/rr-2, a letter to the British Medical Journal by Josh Guetzkow, a senior lecturer at Hebrew University in Jerusalem, in response to the BMJ article, “Covid-19: Researchers face wait for patient level data from Pfizer and Moderna vaccine trials.” Prof. Guetzkow points out that the initial clinical trials doses of the Pfizer-BioNTech modRNA COVID-19 “vaccine”, BNT162b2, were made using what was called “Process 1”, from May to October, 2020; but that the company suddenly changed to a new method, called “Process 2” [by 29 October 2020.] He also points out that the “Process 2” batches of BNT162b2 were found to have “…substantially lower mRNA integrity.” It appears that “substantially lower mRNA integrity” includes evidence of what may be termed “fragments” of DNA appearing in these “vaccines”, where they should never be.
Please bear with Yours Truly, this may get a little “technical”, but it is necessary to the whole.
So, what exactly happened by October 2020 that led Pfizer-BioNTech to change to “Process 2” for BNT162b2? One hint is found on Page 54 of the “Protocol Amendment 9, 29 October 2020” document that the company gave to the FDA (www.nejm.org/doi/suppl/10.1056/NEJMoa2034577; scroll down to “Protocol PDF” and click to get the entire document):
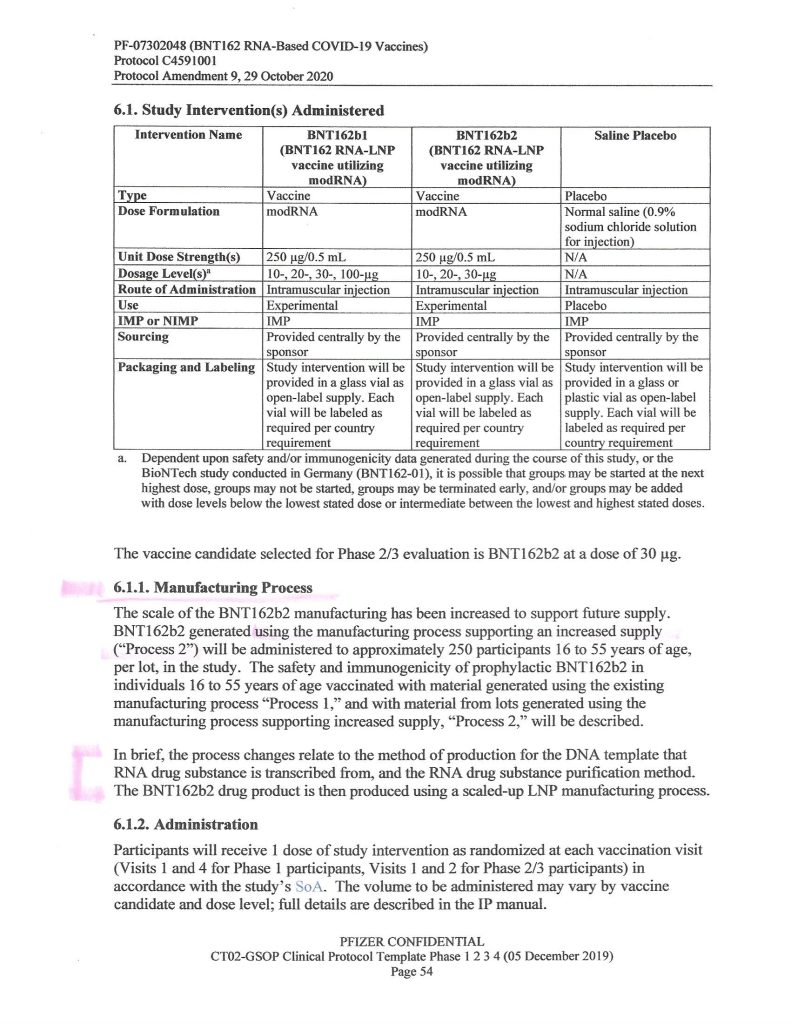
Another hint is found on Page 3 of the same document:
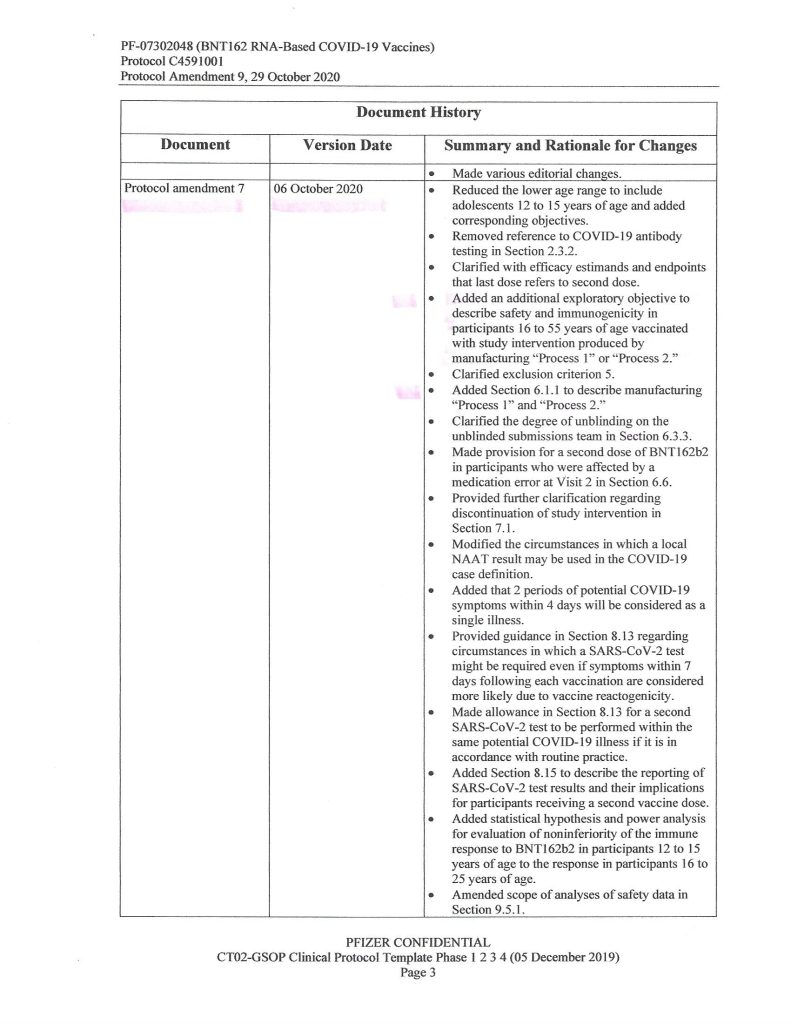
It would appear, then, that Pfizer-BioNTech decided, sometime between 1 May and 1 October 2020, to, One: add an “additional exploratory objective” to the C4591001 clinical study of BNT162b2;, and, Two, to support “increased supply” (of BNT162b2, presumably after securing Emergency Use Authorizations from the European Union medicines regulatory agency and from the FDA in the United States to use the “vaccine”, which did happen) — both, by changing from “Process 1” to “Process 2” to manufacture the product. Prof. Guetzkow states that there appears to be no analysis of comparisons between using these two methods. It is also, from what Yours Truly has been able to find, not known exactly when “Process 1” was stopped as a manufacturing method for BNT162b2 and “Process 2” was approved as the sole method.
We now turn to Page 4 of the FDA-issued “Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum” of 23 June 2023, related to the EUA the agency granted for the use of the Pfizer-BioNTech “2023-2024 Formula” COVID-19 “vaccine” on people ages 6 months to 5 years old in the United States (www.fda.gov/media/172019/download.) Page 4, in Yours Truly’s opinion, is a tacit admission that this “vaccine” is indeed made according to “Process 2”:

Finally, there is the following hint in the description of the manufacturing process for the Pfizer-BioNTech “vaccine” against meningitis, called PENBRAYA, in the 11 DESCRIPTION section of the company-issued document (https://labeling.pfizer.com/ShowLabeling.aspx?id=19937):

The work of Dr. Kevin McKernan, Dr. Jessica Rose, and other researchers, has shown that there are numerous serious issues with the integrity of the process of manufacturing BNT162b2 — the “Process 2” that was used to make billions of doses of this Pfizer-BioNTech modRNA COVID-19 “vaccine” — and which process is being used to manufacture the company’s latest modRNA COVID-19 “vaccine”, the “2023-2024 Formula.” These two scientists, along with other colleagues, published a paper on this situation last month. It can be found here: www.researchgate.net/publication/374870815. The paper also has some more details of the “Process 2” method — and, by the way, also stating that Moderna also came up with a similar process for its modRNA COVID-19 “vaccines.”
One suspects that the integrity problems with the manufacturing of the modRNA COVID-19 “vaccines” is a subject that will have much more investigation. This is aside from the accumulating evidence that the ingredients of said “vaccines” are themselves dangerous.
Peace, Good Energy, Respect: PAVACA







i really appreciate the work you put into this. Where else would we find this amount of information? Definitely not from anyone in the Pravda news.
I am seeing the latest vax from Moderna, the “Spikevax” there calling it pushed a lot now. Wonder how they came up with that name???
After reading the following and noting the DATES, I want to add some useful background.
When going from concept to mass distribution, there are three different steps. As I have found out thru my career, skipping step two can have dire consequences, like blowing up a full scale chemical reaction vessel. Luckily I was on vacation when that piece of idiocy happened. I am going to use that bit of stupidity, by a PhD chemist as an illustration.
These are the steps:
LAB BENCH
During this phase several different formulations are produced for testing. Generally we are talking grams up to maybe a pound of product produced by highly trained people with very good control over the process. For example, if the process is highly exothermic — produces massive amounts of heat — the chemist can stick the flask in a dry ice and acetone bath to keep it from exploding (I did say idiocy above, didn’t I?)
PILOT PLANT
This is the step bean counters LOVE to skip because it costs a LOT of money. This is where the engineers get involved. They are the ones who will see cooling down a highly exothermic process when it is producing 100s of pounds of product is going to be an engineering nightmare. Essentially you are trying to cool down a BIG BOMB.
The engineers during this phase come up with the actual method factory workers will be using to produce the consumer product. This includes not only the design of the equipment, written instructions, but also the ‘check points’ and the needed tests and the test methods used to produce a consistent product. They also determine the specifications & tolerances.
This is the critical step where all the bugs get ironed out IF YOU ARE DEALING WITH A WELL RUN COMPANY.
FULL SCALE PLANT PRODUCTION
Generally the first few production batches are watched very closely by the Chemists, Chem Engineers, Quality Engineers and others. It is the final check on the new process and product to make sure everything is going as planned and the Product is as designed.
>>>>>>
Now that you have a bit of background on how chemical/pharmaceutical plants operate, we can look at “Process 1” and “Process 2” Any bets that “Process 1” produced the better more consistent product BUT WAS NOT SCALEBLE TO PRODUCTION QUANITITIES?
“Process 1” produced the material used for all the lab/FDA testing. It was probably a Lab Bench or very small pilot plant process. I have not done vaccines but I do not think you would need more than about 10 to 20 pounds of material for a vaccine. I was producing about two hundred pounds for FDA testing of a codeine cough medicine.
Remember they were having stability problems early on and the vaccine had to be kept very cold? That may have been “Process 1” material.
Gail Combs
Thank you for this information.
One suspects that somewhere during the early spring of 2020, the top brass of Pfizer-BioNTech (and possibly also of Moderna) concluded that, since the COVID-19 virus was spreading so far and so fast, that a lot more potential COVID-19 “vaccine” production was going to be necessary. This was likely on top of the hysteria and fear caused by the lockdowns and other restrictions being put into place in the United States and other countries.
In other words, Pfizer-BioNTech (and possibly also Moderna) saw a chance to make huge amounts of profit. From there, it is possible that certain “safety measure steps” were short-cutted to change over from “Process 1” to “Process 2” in the “vaccine” manufacturing process.
While this was going on, a small group of the the C4591001 clinical trials test subjects was being “randomly selected” to get the BNT162b2 that was being freshly made using the new “Process 2.” One suspects that the cost of producing the “Process 2” batches was compared to that of a similar-size test group that got the “Process 1” — and the company’s top brass found that it was cheaper to go with “Process 2.”
The C4591001 clinical trial test results data that was given by Pfizer-BioNTech to the FDA in November 2020, when the company applied for the initial EUA for BNT162b2 to be used in the United States, was likely a MIXTURE of data from BOTH “Process 1” AND “Process 2” test subjects.
Since the FDA was likely rushing the EUA approval timeline for BNT162b2, the agency probably didn’t bother to separate out the data given to it into “Process 1” and “Process 2” results. The agency simply granted the initial EUA for BNT162b2 in December 2020.
One suspects that, by possibly August 2020, all “Process 1” manufacturing was stopped, and “Process 2” was substituted as the sole manufacturing method — and the Pfizer-BioNTech is still using it today to make COVID-19 “vaccines”
But, moving on from there —
For argument’s sake, assume that the company has, by now, found the reasons and source of the DNA contamination in the “Process 2” method, and has corrected the issue.
Even if that were the case, that STILL leaves the situation of the DANGEROUS and DEADLY ingredients of the “vaccine” formula ITSELF (SV40 cancer promoter; the ALC-0159 and ALC-0315 dangerous lipid nanoparticles; and the mechanisms of the ACTUAL ingredients of the modRNA used in the “vaccines.”
I’d bet Process 1 is still in use for “special batches” of vaxx for elites. Just my humble opinion.
or just saline decoys to fool the sleeping masses
I vote HCQ/Ivermectin PLUS Saline injections..
Good guess!
excellent, real world insights! TY
I added the title & URL of this article to the previous article The COVID-19 Virus and the modRNA COVID-19 “Vaccines” Induce Accelerated Aging
I will continue using the older article to archive as much of the Covid Vaccine Info that I can.
Gail Combs
Thank you, you are very kind.
Pavaka, thank you for your dedication and hard work to get the information out there. Also thanks to Gail.
It’s amazing that all this information can be kept up with in the glut of information being tossed about.
As always, getting the important information here at Q tree, plucked from all the muck and mire of the cabals deceitful labyrinth and cleaned up presentable like for our examination.
Thank You Pavaca.


Now we wait for the next twists and turns for what’s to be revealed next, by those best able to keep up, like yourself.
Well, looky here — a couple of “finds” —
First:
http://www.cvdvaccine-us.com/6mo-11yearsold/overview
This is the “professional use” overview of the Pfizer-BioNTech “2023-2024 Formula” COVID-19 “vaccine.” This document is for healthcare professionals who will administer this “vaccine” to BABIES 6 months old through CHILDREN 11 years old.
Second, an amazing find, IMO, linked from the one above:
https://webfiles.pfizer.com/Product_Safety_Data_Sheet
This is the Product Safety Sheet for the INGREDIENTS of the Pfizer-BioNTech “2023-2024 Formula” COVID-19 “vaccine” for all age groups.
The Safety Data Sheet lists BOTH the lipid nanoparticles ALC-0159 and ALC-0315 as “Not classified as hazardous” — except that they ARE hazardous, per the Safety Data Sheets on these from Cayman Chemical.
ALSO — Yours Truly suspects that any ingredient listed under a PF- description IS likely to be the ACTUAL formulation of the modRNA used in the “vaccine.” AND, it appears that a DIFFERENT PF- formula is used for DIFFERENT age groups.
One suspects that “PF-” stands for “Proprietary Formula” regarding the exact modRNA ingredients for each age group in the “2023-2024 Formula” COVID-19 “vaccines.”
TY I was wondering. You are doing Amazing Work as you continue to dive into the weeds & expose the truth about the genocide jabs–Thank You!!!
YES – I think you got that right.
PF-07963164 is almost certainly the modRNA which is encapsulated into lipid nanoparticles which are made up of 4 components that are listed, and potentially other “trade secrets” which could include, potentially, PEGylated graphene oxides, etc., as a secret 5th or higher component of the lipid nanoparticles.
Here are the 4 components of the (mixed) lipid nanoparticles:
ALC-0315
ALC-0159
cholesterol
1,2-Distearoyl-sn-glycero-3-phosphocholine
The mixture is buffered by two substances:
tromethamine
Tris(hydroxymethyl)aminomethane hydrochloride (which is just tromethamine HCl)
Then water and sucrose.
What’s fascinating is that I can find absolutely NOTHING about PF-07963164. It is as if that has NEVER been on the internet.
I did run into ANOTHER link that people may find interesting.
https://www.fda.gov/media/144246/download
What a treasure trove of information on the subject. Great work, PAVACA and Gail. Thank you.
I am going to get some good mileage with folks on this with the increased understanding and information. They are going to be spinning their wheels to get away from me.
Adding this one in that was forwarded by Dr. Malone today. Statistical analysis.
https://drreidsheftall.substack.com/p/how-many-people-died-in-the-us-during?utm_source=cross-post&publication_id=574407&post_id=138621435&utm_campaign=583200&isFreemail=true&r=1p1fzp&utm_medium=email
Just going to mention NVIC (National Vaccine Information Center NVIC.org) for any who would like the information. It was founded by Barbar Loe Fisher, who had a vaxx injured son. They are being highly sensored at the usual places, and they need our support. Maybe someone would kindly post the link.
https://www.nvic.org/
https://www.nvic.org/
Hey, Barkerjim and Kalbo, thanks so much for the help, and hi, Wolfmoon and everyone who sees this.
singularzoe
Greetings to you and hope that all is well.
Nice to see you at the best Tree Zoe! God Bless YOU Bigly 
Pavaca, I am well, thank you. Hope you are the same.
Valerie, God bless you, also. So nice to have you as a sis in Christ.
From GA/FL
ANOTHER COVID FIGHTER!
We know about
Quercetin
HCQ
Ivermectin
Nattokinase
+Zinc and D3
and now:
Artemesia/Wormwood
—
The Vigilant Fox @VigilantFox
This Nobel Prize-Winning Drug Is Now Quietly Under Attack: It’s Not Hydroxychloroquine or Ivermectin Enter Artemisia Annua (Artemisin, Sweet Wormwood), a cheap, safe, ancient herbal medicine and anti-malarial that also treats COVID-19. But that’s not all it does: • broad antiviral activity against viruses (Herpes, HIV, Hep B&C, EBV, CMV, Zika) • binds Spike protein of SARS-COV2 more strongly than hydroxychloroquine • has immunoregulatory effects to decrease cytokines, cytokine storm, ARDS, organ damage and lung fibrosis in COVID-19 infection (blocks NF-κB signaling) • Anti-cancer affects (via blocking NF-κB) for prostate, cervical, colorectal cancers However, papers on the drug have been retracted. Herbal products recalled. Why is it under attack? This information was compiled by an article written by Dr. William Makis – *https://makismd.substack.com/- *https://twitter.com/MakisMD
The article by Dr William Makis:
Artemisia Annua (Artemisin, Sweet Wormwood) – NOBEL PRIZE WINNING top anti-malarial that also treats COVID-19. Papers retracted, herbal products recalled. Quietly under attack. WHY?
you get a few days free and then you must subscribe.
He does have this info for free:
Source: NOVARTIS – “In China, Novartis works with about 100,000 farmers who cultivate Artemisia Annua, a crucial plant in the production of our antimalarial treatment.”
Papers reviewed:
>>>>>>>>>>>>>>>>>
Artemisia Annua, Artemisinin & 2015 Nobel Prize in Medicine – A Cancer Fighting Plant
For us this is important
(PDF) Cultivation of Artemisia (Artemisia annua Linn.)
researchgate.net›
Characteristics of strain(s) for cultivation
Then follows instructions for cultivation and harvesting.
This is interesting. A group working with and for Africans
https://maison-artemisia.org/en/association/#histoire
Feedback Regarding The Use of Artemisia annua With a Wide Range of Health Problems