The above image is of mass vaccination against smallpox in Paris in 1905. (Courtesy, Getty Images.)
Today’s post will trace what ** may be ** a “sleight-of-hand” that started out with Xavier Becerra, the Secretary of the United States government Department of Health and Human Services, giving the “Go-Ahead” for the use of the H5N1 Avian Influenza “vaccine”, AUDENZ, in anticipation of a potential “bird flu pandemic” in the United States; but, which since has been “transformed” into the American Medical Association just issuing new CPT codes for an Avian Influenza “vaccine” for a different strain, called H5N8. Meanwhile, the CDC / FDA / United States government, are all sending out warnings related to the H5N1 strain. Stay with Yours Truly, it gets even better — “Mais, mon Dieu!” — the twists and turns! This post is a kind of “snapshot” of the situation — it is an evolving issue.
For purposes of today’s post, the trail begins here: www.ernst.senate.gov/imo/media/doc/fowl_play_squeal.pdf, the letter that Sen. Jodi Ernst (R-Iowa) sent to USDA Secretary Tom Vilsack on 14 February 2024. In this letter, Sen. Ernst demands answers regarding United States government funding of what appears to be Gain-of-Function research experiments on Avian Influenza viruses; which experiments involve a scientist linked to the Chinese Communist Party. Yours Truly can find to date, no response from Sec. Vilsack to Sen. Ernst. A screenshot of Page 1 of her letter is below:

Yours Truly now turns to this: www.aha.org/news/headline/2024-07-23-hhs-broadens-emergency-declaration-facilitate-response-bird-flu-other-viruses-pandemic-potential, “HHS broadens emergency declaration to facilitate response to bird flu, other viruses with pandemic potential”, dated 23 July 2024, which “expanded” the 2013 amendment to the Federal Food, Drugs, and Cosmetics Act to now include “other viruses” that may have “pandemic potential.” The document, from HHS Secretary Xavier Becerra, specifically mentions three types of Avian Influenza strains: H1N1 (from 2009); H7N9 (from the 2013 amendment);, and H5N1 (from the 24 March 2024 USDA statement regarding H5N1 infections in dairy cows in Kansas and in Texas.) https://public-inspection.federalregister.com/2024-16247.pdf. Below is a screenshot from the AHA (American Hospital Association) press release:

And, for reference, here is the Congressional Research Service Legal Sidebar document related to what the HHS Secretary can “declare” under the PREP Act (including removing liability options), updated 21 July 2023: https://crsreports.congress.gov/product/pdf/LSB/LSB10730, “The PREP Act and COVID-19, Part 2: The PREP Act Declaration for COVID-19 Countermeasures.”
This was followed by a tweet from Robert Kennedy, Jr.: https://twitter.com/RobertKennedyJr/status/1816905031653675473, a screenshot of which follows:

Meanwhile, the USDA had already issued a press release regarding how dairy farmers can apply to receive expanded livestock assistance to compensate for milk production lost due to their cows infected with H5N1: www.usda.gov/media/press-releases/2024/06/27/usda-begin-accepting-applications-expanded-emergency-livestock, “USDA to Begin Accepting Applications for Expanded Emergency Livestock Assistance Program to Help Dairy Producers Offset Milk Loss Due to H5N1”, dated 27 June 2024.
Which was followed, in turn, by a CDC release regarding the government’s response to the current H5N1 Avian Influenza situation: www,cdc,gov/bird-flu/spotlights/h5n1-response-07262024.html; a screenshot from the release is below:

So, it would appear that the HHS gave the “go-ahead” for a kind of “EUA” regarding the use of the protein-subunit H5N1 “vaccine”, AUDENZ (a supply is already in the National Vaccine Stockpile); and, for the increased production of this “vaccine.” What follows is a “closer look” at AUDENZ. Yours Truly will begin with the FDA-issued Fact Sheet for healthcare providers for this “vaccine”: www.fda.gov/media/135020/download; three screenshots from the document are below. The first screenshot shows clearly that there was no Placebo group (Control/saline group) in at least two clinical trials for AUDENZ. The second screenshot shows clearly that no Toxicology studies were performed for AUDENZ. The third screenshot is Page 10 of the Fact Sheet.
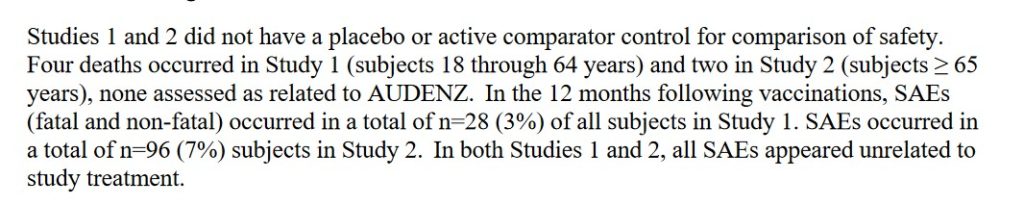


Note this language in the first screenshot: “In both Studies 1 and 2, all SAEs appeared unrelated to study treatment.” This indicates at least two important details: One, that Serious Adverse Events (SAEs) did occur during at least two clinical trials of AUDENZ; and, Two, that these Serious Adverse Events were not considered to be related to the clinical trials for AUDENZ. This is the same type of language that Pfizer-BioNTech used regarding Serious Adverse Events that occurred during the (shortened and data-compromised) clinical trials for the company’s “flagship” COVID-19 modRNA “vaccine” BNT162b2. Note also that a “complete dose series” for AUDENZ is two separate doses of 0.5mL each, for all age groups age six months and up. AUDENZ uses an adjuvant (an ingredient that facilities the activities of the injectable) called MF59. MF59 is a squalene-based, oil-in-water adjuvant. The Safety Data Sheet for MF59 is here: https://file.medchemexpress.com/batch_PDF/HY-153206/MF59-SDS-MedChemExpress.pdf. The product is listed as “Not a hazardous product or mixture” in section 2.2 of this document. However, reading further down the same document, one finds all sorts of contradictory information in the sections on “First Aid Measures”, on “Handling and Storage”, on “Exposure Controls”, and more.
Note also the list of reported adverse events in section 6.2 of Page 10, above. These are same types of adverse events reactions to the Pfizer-BioNTech modRNA COVID-19 “vaccine”, BNT162b2, in the post-authorization report that this company gave to the FDA on 30 April 2021; and in reports to VAERS; and, which are listed within the FDA-issued Fact Sheet for Healthcare Providers for the “2023-2024 Formula COVID-19 Vaccine” by Pfizer-BioNTech (www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf; www.openvaers.com/; www.fda.gov/media/167211/download?attachment. Very troubling are the listings in section 6.2 of Page 10 above in the AUDENZ fact sheet for “convulsions”; “demyelination”; “encephalitis”; and, “Guillain-Barre’ syndrome.”
A blog post by Dr. Jessica Rose, PhD, on 27 June 2024, presents a summary of clinical trial for AUDENZ (NCT02839440), in which she proves that the fatality rate is 1/200 chances for AUDENZ: https://jessicar.substack.com/p/1200-chance-of-death-in-context-of, “1/200 chance of death in context of new bird flu injection – 5 times higher than placebo according to clinical trial.” NCT02839440 did have a Placebo control group, (Scroll down the blog post to the discussion of this clinical trial for AUDENZ.)
To date, Yours Truly can find no exact set of CPT Codes for AUDENZ. The closest item found is here: www.hhs.gov/guidance/document/flu-shot-coding-0; the listing is “Q2039 Influenza virus vaccination otherwise specified.”
AUDENZ is produced by CSL Seqirus, part of the much-larger CSL multinational drug company. Following is a JPG of the list of CSL offices and locations, sourced from: www.csl.com/:

The United States government awarded CSL Seqirus a contract to produce millions of doses of an Avian Influenza “vaccine” in May 2024: www.cslseqirus.us/news/csl-seqirus-announces-us-government-award-in-response-to-avian-influenza. The “vaccine” will be manufactured by the CSL Seqirus facility at Holly Springs, North Carolina. This facility was built in partnership with BARDA (Biomedical Advanced Research and Development Authority), a department of the United States government. The Avian Influenza “vaccine” that this facility will manufacture is “cell-based”, as opposed to “egg-based”; with a six-month “turnaround” for production: www.csl.com/we-are-csl/our-business-and-products/csl-seqirus/csl-seqirus-manufacturing-technologies. Below are two images from the article related to this facility:


Note that whatever Avian Influenza cell-based “vaccine” from the CSL Seqirus facility at Holly Springs will use the MF59 adjuvant. (By the way, MF59 is trademarked by Novartis AG, which was acquired by CSL.)
So far, it appears that the H5N1 strain of Avian Influenza is the one that the United States government is focused upon. However, here’s where the trail veers to another path.
Please refer back to the American Hospital Association press release above in today’s post. Note this language: “The amendment now applies to pandemic influenza A viruses and others with pandemic potential, such as the current H5N1 strain of bird flu…” (Italics, Yours Truly) In Yours Truly’s opinion, this is “a hole big enough to drive a truck through” — or, perhaps, another strain of Avian Influenza.
The following article beings to “lift the curtain” on what ** may ** really be going on — which, again in Yours Truly’s opinion, appears to be a kind of “sleight-of-hand”: www.naturalnews.com/2024-07-26-fda-grant-eua-mrna-bird-flu-vaccines.html, “Pandemic 2.0 ready to go: FDA to grant emergency use authorization (EUA) to mRNA bird flu shots, just like what happened with COVID“, by Ethan Huff. And, “right out of the gate”, the article begins with this:

But, wasn’t the “upcoming potential bird flu pandemic” supposed to be the H5N1 strain that the government is warning about? Where does the H5N8 strain come in? According to Wikipedia, the H5N8 strain of Avian Influenza is “is typically not associated with humans.” https://en.wikipedia.org/wiki/Influenza_A_virus_subtype_H5N8. Very few humans have contracted a case of H5N8; this virus strain predominates among wild birds. However, the mortality rate among wild birds infected with H5N8 is “at least 75%”, again according to the Wikipedia article above.
Now, turning to the American Medical Association’s issuing new CPT Codes for the use of an H5N8 “vaccine.” One media outlet that Yours Truly found has this: https://revcycleintelligence.com/news/ama-updates-cpt-code-set-for-avian-influenza-vaccines, dated 22 July 2024. Below is a screenshot from the article, with the new CPT codes:

Note that the American Medical Association owns the rights for the CPT codes. This means that the AMA gets a “royalty payment” every time a CPT code is used. The AMA notice regarding the CPT codes for H5N8 is here: www.ama-assn.org/press-center/press-releases/ama-announces-cpt-update-avian-influenza-vaccines, dated 19 July 2024. Below is a screenshot from the notice:

What’s going on here? The picture is, to say the least, somewhat “murky.” There are, however, a few potential clues. Among them is this: www.pennmedicine.org/news/news-releases/2024/may/penn-researchers-develop-experimental-mrna-avian-flu-vaccine, dated 23 May 2024. Note on the screenshot, below, from the article, the language regarding “a specific type of the H5N1 virus”; and, that animals other than wild birds were being used for the experiments:

Here is another clue, from 5 June 2024: www.idsociety.org/science-speaks-blog/u.s.-orders-4.8-million-doses-of-a-cell-based-adjuvanted-h5-vaccine-for-avian-flu-preparedness#/+/0/publishedDate_na_dt/desc/, by Daniel R. Lucey, MD, PhH, FIDSA. Below is a screenshot from this article:
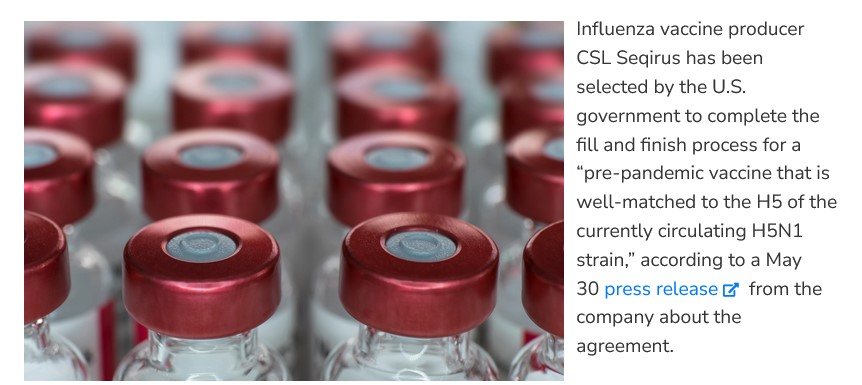
Note the language regarding “pre-pandemic vaccine that is well-matched to the H5 of the currently circulating H5N1 strain,…” (bolding, Yours Truly)
A third clue is here: https://twitter.com/RenzTom/status/1816110256843264368; a screenshot of part of his tweet is below:
And, a fourth clue is here: https://clinicaltrials.gov/ct2/show/NCT05874713, a clinical trial that appears to be in the “wrapping-up” stages regarding testing an mRNA-based H5N8 “vaccine.” Below is a screenshot from the Clinical Trials webpage for this clinical trial:

Note the very low test subject enrollment (480 persons); the presence of the MF59 adjuvant in the H5N8 “vaccine” candidate used in the clinical trial; and, a “two-dose” series of “primary run” injections of the “vaccine” candidate, followed by a “booster shot” on Day 209 for the H5N8 “vaccine” candidate.
There are three other clinical trials of an H5N8 “vaccine” listed on the https://clinicaltrials.gov/ website: NCT02624219 (Completed); NCT05975840 (Active); and, NCT03014310 (Completed.) All of these clinical trials were/are Phase I or Phase I/II. None of them have a Placebo/saline control group. Each of them have fewer than 600 subjects (NCT02624219 had 275 test subjects.) Two of these three other clinical trials have the NIAID as the Sponsor.
What does all this mean? Is it possible that the current H5N1 “Avian Influenza infecting cattle, cows, and domesticated pets, in addition to poultry” situation, while it is indeed occurring, is also a sort of “Look, squirrel!” to distract from something that may be more dangerous?: from, perhaps, Gain-of-Function experiments on the H5N8 strain of the Avian Influenza (recall that this strain has a 75%+ mortality rate among the wild birds that it infects); plus, perhaps. the development of “vaccines” for this “perhaps-enhanced” H5N8 strain, which may include the millions of doses of an “Avian Influenza cell-based vaccine” that will be manufactured at the CSL Seqirus facility in North Carolina? In other words, “Pandemic 2.0”?
Good Energy, Peace, Respect: PAVACA







Nicely done!
I have a bad feeling this may come in handy.
Coothie
Thank you, you are very kind.
Yours Truly agrees that the information in the above post “may come in handy.”
From the opener today by our good DePat:
https://twitter.com/toobaffled/status/1819171984301051955
And, regarding “vaccine fanatic”, Dr. Peter Hotez (this was presented by Yours Truly on the main board thread a few days ago):
https://twitter.com/newstart_2024/status/1816109385740956137
And, from the AMA regarding “anti-science aggression” and “anti-vaccine aggression”:
http://www.ama-assn.org/delivering-care/public-health/dr-peter-hotez-what-feeds-vaccine-refusal-anti-science-aggression
14 December 2021
http://www.ama-assn.org/delivering-care/public-health/dr-peter-hotez-anti-science-movement-and-declining-joe-rogan-s-debate
13 July 2023
And, from the AMA, giving “tips” to physicians regarding how to “fight anti-science aggression” in order to get patients to take the COVID-19 “vaccines” (or, for that matter, any vaccine):
http://www.ama-assn.org/delivering-care/public-health/5-tips-battle-anti-science-aggression-doctor-front-lines
26 July 2023
Yours Truly is now going to make a prediction here. One predicts that “anti-science aggression” and/or “anti-vaccine aggression” can, perhaps sooner rather than later, be “labeled” by HHS / CDC / FDA, as “public health emergencies” under the PREP Act. This could, potentially, make anyone who is skeptical of “vaccination” and/or who refuses “vaccination” (either for themselves or for their family members — on religious grounds; or as “conscientious objectors”; or from having studied the increasing amount of published studies that show how dangerous the modRNA “vaccines” are; and/or are following an alternative medicine protocol), into a criminal.
https://www.zerohedge.com/markets/moderna-shares-tumble-12-after-full-year-sales-guidance-slashed
“About a month ago, the Biomedical Advanced Research and Development Authority (BARDA) granted Moderna $176 million to develop bird flu vaccines. Now, Moderna is banking on a spike in human-to-human bird flu cases.”
Great report, TY for all the time and effort you dedicate to bring this to everyone!
$176m for RD, how convenient is that? So I guess it’s the responsibility of the DHHS to create a new pandemic now. Wink, Wink, to big pharm. The money will be flowing to the usual subjects now.
CV sets it all up, I can just contribute pieces that fit into her framework.
Coothie
Thank you for this. It is confirmation of what Yours Truly presented on the main discussion thread of the board a few weeks ago.
The award was originally broadcast as being given for Avian Influenza “vaccine” development (no awardee was named.) Then, it was revealed that the award was ONLY intended for Moderna.
Thanks for all the work and heads up on this, PAVACA!
TradeBait2
Thank you, you are very kind.
We’ve come to a vicious deadly cycle.
-our science community develops a pathogen
-the pathogen escapes from the lab
-our politicians vote to pay their crony pharmaceutical companies to develop a ‘vaccine’ to fit the pathogen
-the pharmaceutical companies make billions of $$$
-the pharmaceutical companies give a heap of that money back to our politicians in the form of campaign donations
-our politicians and health agencies then (try to) force everyone to take these ‘vaccines’
The formatting was not very cooperative
Much like MIC AND Endless Wars.
Formatting. Looking good on my end.
GA/FL
Thank you, you are very kind.
It is a kind of dreadful “wash, rinse, repeat” cycle.
I think we have the most comprehensive and up-to-date information here, thanks to you, Pavaca!
TheseTruths
Thank you, you are very kind.
And, thank you for all the things you bring to the board regarding COVID-19 and the “vaccines.”
Thank you, PAVACA!
barkerjim
You are very kind. Keeping up with this situation is not a “walk in the park”, for sure. Thank you for all the items you bring to the board about COVID-19 and the “vaccines.”